Medullary Thyroid Cancer (MTC)
 Fig.1: Thyroidectomy specimen of MTC showing characteristic involvement of both lobes, and yellow cut surfaceMedullary cancers (MTC) are the third most common form of thyroid cancer, comprising approximately 4% of the total. MTC was not recognised as a disease in its own right until 1959 when it was described by Hazzard and others, who reviewed 600 cases of thyroid carcinoma at the Cleveland Clinic over a 31-year period and identified 21 tumours with unique histological features, which they called medullary thyroid carcinoma (Fig. 1).
Fig.1: Thyroidectomy specimen of MTC showing characteristic involvement of both lobes, and yellow cut surfaceMedullary cancers (MTC) are the third most common form of thyroid cancer, comprising approximately 4% of the total. MTC was not recognised as a disease in its own right until 1959 when it was described by Hazzard and others, who reviewed 600 cases of thyroid carcinoma at the Cleveland Clinic over a 31-year period and identified 21 tumours with unique histological features, which they called medullary thyroid carcinoma (Fig. 1).
In contrast to PTC, the incidence of MTC is not increasing, so the proportion of all thyroid cancers being medullary in type continues to drop.
Unlike PTC and FTC, medullary cancers arise from a group of cells within the thyroid called parafollicular cells (or C cells), which produce a hormone called calcitonin.
When there is an increase in the C cells (C cell hyperplasia) or if the C cells become cancerous (medullary thyroid cancer), large amounts of calcitonin are detectable in the blood.
The calcitonin level acts as an indicator of cancer activity (it is a tumour marker, so the higher the calcitonin the greater the tumour bulk). C cells do not concentrate iodine so radioactive iodine is of no value in the management.
Presentation of MTC
Sporadic (non-genetic) MTC usually presents between the fourth and sixth decade of life. Like most thyroid tumours, MTC can present with a lump in the thyroid, hoarse voice, difficulty swallowing (dysphagia), lymph nodes in the neck or distant metastases. Females are more commonly affected than males (except for inherited cancers) and it is not associated with previous radiation exposure.
MTC tends to spread to neck lymph nodes early in the disease, but spread to distant organs occurs late (to the liver, bone, brain, and adrenal medulla). Central and lateral compartment lymph node metastases are present respectively in 14% and 11% of patients with T1 tumours, and in 86% and 93% of patients with T4 tumours.
Unfortunately, 70% of patients with MTC who present with a palpable thyroid nodule have cervical metastases and 10% have distant metastases at presentation. The rates of nodal involvement with tumour size (T-stage) are listed below, and in addition higher calcitonin levels make lateral compartment nodal involvement more likely:
- T1 - ~14%
- T2 - 50%
- T3-4 - ~90%
The clinical course of patients with MTC is variable, ranging from indolent to extremely aggressive, and it is related to the stage of the disease at the time of diagnosis. Survival rate at 10 years is 90% for patients with tumours limited to the thyroid, 70% for patients with involved nodes in the neck, and only 25% when spread has occurred to other sites in the body. Patients who undergo thyroid surgery for C-cell hyperplasia (which is the precursor to MTC) have a survival of 100%.
Poor prognostic factors include age >50, male, distant spread (metastases), and when seen in patients with other endocrine tumours due to MEN 2B syndrome. Ten-year survival rates for patients with stages I, II, III, and IV MTC are 100%, 93%, 71%, and 21%, respectively. The clinical behaviour of sporadic MTC is unpredictable; however, some patients with distant metastases may live for several years. In recent decades there has been no significant trend toward an earlier stage of disease at the time of diagnosis, as just under half of the patients present with stage III or IV disease. Also, there has been no significant increase in patient survival.
There are four main ways that medullary cancer is seen:
1. Sporadic
This accounts for 75-80% of all cases of MTC, with no evidence of other endocrine conditions or familial history. They typically present with a unilateral mass in the thyroid, with a peak onset between 40 – 60 years and with a 1.5x preponderance of females. Patients tend to be older and have a higher calcitonin level. One third will present with intractable diarrhoea caused by excessive calcitonin secretion.
2. Familial MEN Type 2A (MEN2A)
This syndrome (see MEN2 syndromes) comprises bilateral MTC or C cell hyperplasia, an adrenal tumour called a phaeochromocytoma, and hyperparathyroidism. It is caused by an inherited genetic defect in the RET gene on chromosome 10 and can affect males and females equally. Peak incidence of MTC in MEN2A patients is between 30-40 years.
Molecular testing of relatives allows individuals to be screened and to predict who will develop cancer. This allows prophylactic surgery to be performed on children with the genetic mutations.
3. Familial MEN Type 2B (MEN2B)
This syndrome (see MEN2 syndromes) also comprises MTC and phaeochromocytoma, but only rarely will there be hyperparathyroidism. Instead these patients have an unusual appearance which is characterized by mucosal ganglioneuromas (tumours in the mouth) and a Marfanoid habitus (tall stature with long arms).
This is caused by a mutation in the same RET proto-oncogene that causes MEN2A, but a slightly different type of mutation seems to be responsible. MEN2B patients usually get MTC in their 30's, and males and females are equally affected.
4. Familial (FMTC)
This form of MTC is not associated with any other endocrine tumours, although it is inherited as a subset of MEN2A (see MEN2 syndromes), which distinguishes it from the sporadic case. The peak incidence is between the ages of 40 and 50. Currently, the opinion of most clinical investigators is that FMTC should not be a freestanding syndrome; rather it should represent a variant along the spectrum of disease expression in MEN2A. Premature labelling of a family as FMTC may run the risk of missing a phaeochromocytoma.
A summary of the genetic basis for MTC is given below in Table 1, outlining the correlation between genetic RET codon abnormalities and aggressive disease, along with the incidence of other manifestations of the MEN2 syndromes.
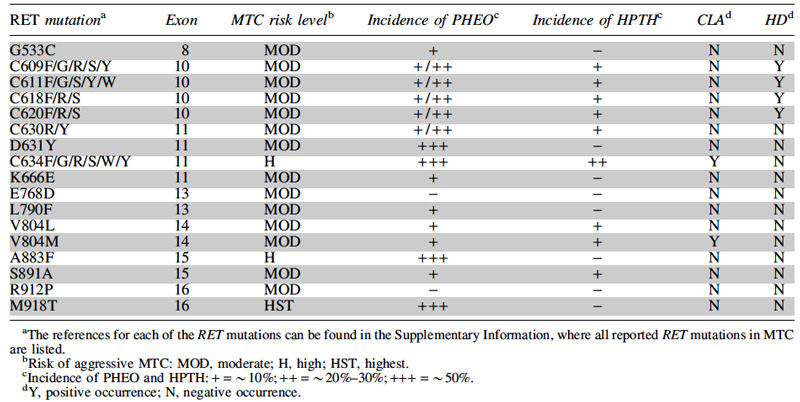 Table 1: Relationship of common RET mutations to the risk of aggressive MTC in MEN2A & 2B, and to the incidence of phaeochromocytoma (PHEO), hyperparathyroidism (HPTH), cutaneous lichen amyloidosis (CLA) and Hirschsprung's disease (HD). Source: ATA Guidelines on MTC Management
Table 1: Relationship of common RET mutations to the risk of aggressive MTC in MEN2A & 2B, and to the incidence of phaeochromocytoma (PHEO), hyperparathyroidism (HPTH), cutaneous lichen amyloidosis (CLA) and Hirschsprung's disease (HD). Source: ATA Guidelines on MTC Management
Diagnosis of Medullary Cancer
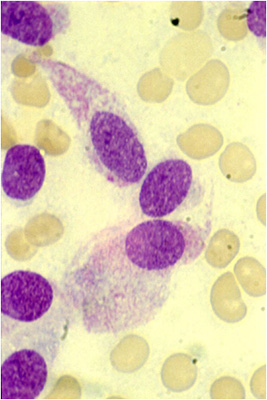 Fig.2: MTC cytology with spindle shaped cells & granular cytoplasmMTC is diagnosed by a combination of clinical suspicion, FNA cytology, and a blood test for calcitonin and CEA (which is also often elevated in MTC).
Fig.2: MTC cytology with spindle shaped cells & granular cytoplasmMTC is diagnosed by a combination of clinical suspicion, FNA cytology, and a blood test for calcitonin and CEA (which is also often elevated in MTC).
FNA cytology (Fig. 2) shows MTC cells to be usually dis-cohesive or weakly cohesive, and they may be spindle-shaped, plasmacytoid, or epithelioid. Up to 25% of FNA results will not be malignant however, but will usually contain atypical features.
The diagnosis of MTC can be verified by staining for calcitonin (Fig. 3), chromogranin, or CEA; by confirming lack of thyroglobulin staining; and most importantly by detecting elevated serum Ctn levels in the patient.
Basal levels of serum calcitonin and CEA should be measured concurrently. In patients with advanced MTC, a marked elevation in the serum CEA level out of proportion to a lower serum calcitonin level, or normal or low levels of both serum calcitonin and CEA indicate poorly differentiated MTC.
In very early disease (such as in screening for familial syndromes), levels of calcitonin may not be raised, but may be stimulated either by calcium or pentagastrin. It is suggested that borderline baseline levels are further investigated by a stimulation test.
Ultrasound is performed as described for the surgery of a simple goitre, which will determine the extent of disease in the neck and the presence of any lymph nodes. It will also guide whether further imaging is needed preoperatively, if there is extensive disease in the neck.
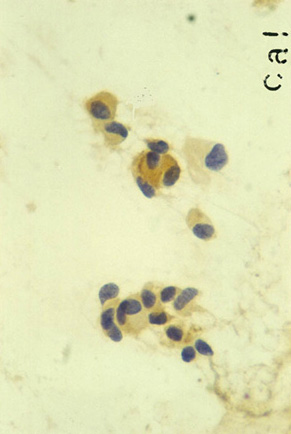 Fig.3: Characteristic positive calcitonin stain of MTC cytology
Fig.3: Characteristic positive calcitonin stain of MTC cytology
CT or MRI scans are recommended in patients with extensive neck disease and signs or symptoms of regional or distant metastases, and in all patients with a serum calcitonin level greater than 500 pg/mL.
FDG-PET CT scanning is less sensitive in detecting metastases and is not recommended in the ATA Guidelines for management of MTC.
A careful family history should be obtained and special importance given to relatives who died from unknown causes at an early age, perhaps during childbirth or while undergoing anaesthesia for a routine operation.
Massive elevations in blood pressure can be caused by the presence of an unrecognised phaeochromocytoma, resulting in a high risk of heart attack or stroke, and even sudden death, especially with external stress such as childbirth or anaesthesia.
So in addition to the standard tests, careful assessment of the adrenal glands for tumour activity is mandatory (24 hour urine collections for catecholamines and blood tests to exclude a phaeochromocytoma). Blood tests for calcium and parathyroid hormone levels will identify the presence or absence of parathyroid tumours. If the blood tests suggest parathyroid or adrenal tumours are present then further imaging tests must be done to localise the tumours.
Tumours of the parathyroid can be dealt with when the total thyroidectomy for the MTC is performed, but patients with phaeochromocytomas must be rendered safe by medication and adrenal tumours removed first, before total thyroidectomy can be carried out.
In summary, the appropriate workup of a medullary cancer patient should include:
- family history
- calcitonin and CEA levels
- calcium, PTH and vitamin D levels
- plasma metanephrines
- neck US with FNA +/- calcitonin levels
- CT of chest/abdomen/pelvis
- laryngoscopy
- genetic testing for RET mutation
Treatment
Total thyroidectomy & Central Neck Dissection
The best chance of curing a patient with MTC is at the first operation, when the appropriate radical surgery should be carried out. The surgery is very similar to that for simple goitre, but is more extensive for a number of reasons: MTC tends to involve both lobes of the thyroid gland, there are multiple tumours in the hereditary forms of the disease, and radioactive iodine is ineffective in this condition.
Patients with MTC need a total thyroidectomy, with removal of all the lymph nodes and tissue in front of the windpipe (level VI), including the thymus (this gland lies behind the breast bone).
Further description of the surgery and lymph node management appears on the webpages Surgery Options and Lymph Nodes in Thyroid Cancer. I have also created a booklet, outlining the steps in diagnosis and treatment of thyroid cancer, which can be found on webpage Guide to Surgery.
Lymph Node Management
By the time of diagnosis up to 20% of MTC patients have developed distant metastases. Lymph node metastases can occur with primary tumours as small as 5mm. Distant metastases can occur with primary tumours only 10mm in diameter.
Very useful information can be gained from the preoperative serum calcitonin and CEA, which can guide the extent of lymph node dissection. The higher the levels, the more likely nodal metastases were present, with the highest levels also more likely to indicate lateral nodal compartment disease. In a 2010 study of 300 consecutive patients with MTC treated by total thyroidectomy and compartment-oriented lymph node dissections, there was virtually no risk of lymph node metastases when the preoperative serum calcitonin level was less than 20 pg/mL (normal reference range < 10 pg/mL).
However, basal serum calcitonin levels exceeding 20, 50, 200, and 500 pg/mL were associated, respectively, with metastases to lymph nodes in the ipsilateral central and ipsilateral lateral neck, the contralateral central neck, the contralateral lateral neck, and the upper mediastinum. As the calcitonin level rises there is an increased risk of contralateral lymph node metastases (CLNM). A calcitonin over 2000 pg/ml has a 60% chance of CLMN, a 30% chance of mediastinal LNM and 40% chance of distant metastases.
Bilateral compartment oriented neck dissection achieved postoperative biochemical cure in at least half of the patients with pretreatment basal calcitonin levels of 1000 pg/mL or less, but not in patients with levels greater than 10,000 pg/mL.
Generally, if there is involvement of 10 or more lymph nodes and more than two compartments of the neck, then cure is near impossible.
Central and lateral lymph node involvement on the same side of the neck occurs equally often, but there is a strong correlation between the number of central nodes involved and the likelihood of lateral node involvement. One to three involved central nodes increases the likelihood of involved nodes in the ipsilateral neck from 10 to 77%, and four or more positive central nodes increase this to a 98% chance.
According to the latest Guidelines of the American Thyroid Association (published in 2015), both familial and sporadic type MTC should have total thyroidectomy with routine dissection of nodes in the central compartment of the neck. In patients with MTC and no evidence of neck metastases on US, and no distant metastases, dissection of lymph nodes in the lateral compartments (levels II–V) may be considered based on serum calcitonin levels, however consensus was not achieved with this guideline.
If central nodes are involved, then at least the lateral compartment on the side of the primary tumour should also be dissected. Patients with MTC confined to the neck and cervical lymph nodes should have a total thyroidectomy, dissection of the central lymph node compartment (level VI), and dissection of the involved lateral neck compartments (levels II–V).
The dissection of the neck should be meticulous and there is no justification for any form of ‘berry picking’. The major structures of the neck should be skeletonised and microdissection techniques should be encouraged. All too often recurrent disease arises either in the central part of the neck or laterally, due to inadequate primary lymph node clearance. Thymectomy should also be regularly performed, as metastatic disease is often present.
Contralateral Node Dissection?
When preoperative imaging is positive in the ipsilateral lateral neck compartment but negative in the contralateral neck compartment, contralateral neck dissection should be considered if the basal serum calcitonin level is greater than 200 pg/mL. For the contralateral neck and the mediastinum, the risk of involvement increases with the size of the tumour, with multifocal MTC in the thyroid, and with the number of involved central nodes. When no central nodes are involved the risk of contralateral nodal metastases is less than 5%, but if there are 10 or more positive central nodes the risk reaches 77% and dissection of the central and both lateral compartments is necessary.
With ipsilateral lymphatic drainage of tumour cells (i.e. involvement of only the central and lateral cervical lymph node compartments on the side of the primary cancer), surgical cure may be attainable in some patients, whereas metastases in the contralateral lateral compartment herald incurable disease.
In the presence of extensive regional or metastatic disease less aggressive surgery in the central and lateral neck may be appropriate to preserve speech, swallowing, parathyroid function, and shoulder mobility. External beam radiotherapy (EBRT), systemic medical therapy, and other nonsurgical therapies should be considered to achieve local tumour control.
Persistent or Recurrent Disease
Occasionally, the diagnosis of MTC is made following a unilateral hemithyroidectomy. The opposite thyroid lobe should be removed in patients with hereditary MTC because the likelihood that MTC is already present or will develop in the future approaches 100%.
In patients with sporadic MTC the incidence of bilateral MTC ranges from 0% to 9%, and there are few studies evaluating patient management in this setting. The ATA Guidelines recommend that following unilateral thyroidectomy for presumed sporadic MTC, completion thyroidectomy should be performed in patients with a RET germline mutation, an elevated postoperative serum calcitonin level, or imaging studies indicating residual MTC. The management of a persistently raised calcitonin following surgery is a difficult problem. There is little evidence that reoperating on patients with mildly elevated calcitonin improves survival. The presence of an enlarged lymph node in association with a normal serum calcitonin level is not an indication for repeat surgery.
In patients having an inadequate lymph node dissection at the initial thyroidectomy a repeat operation, including compartment oriented lymph node dissection, should be considered only if the preoperative basal serum calcitonin level is less than 1000 pg/mL and five or fewer metastatic lymph nodes were removed at the initial surgery. The biochemical cure rate after repeat surgery drops markedly if either of these features are present. The cure rate is <1% when preoperative calcitonin is >1000 pg/ml, but between 40-100% if preop calcitonin is <1000pg/ml. If greater than 5 positive lymph nodes are removed, then cure rate is between 0-6% irrespective of preop calcitonin.
In patients with recurrent disease, further surgical exploration should be considered, but it is rare in these patients to achieve an undetectable calcitonin.
In clinically apparent MTC, systemic disease is common. In patients who have raised calcitonins but occult disease, laparoscopy, bone scanning, MRI, FDG-PET scanning and angiography and venous sampling all have something to contribute to help plan any reoperation contemplated.
Prognosis
It should be noted that patients whose basal serum calcitonin level is normal (< 10 pg/mL) following attempted complete lymph node dissection are said to be ‘‘biochemically cured’’ and have a 97.7% survival at 10 years. However, 3% of patients with a normal baseline serum calcitonin level following thyroidectomy will have a biochemical recurrence within 7.5 years. Even with extensive lymph node dissection, only 60% of node-negative and 10% of node-positive patients are cured. Despite this, the overall prognosis is still remarkably good at around 85% at 10 years.
Doubling time of postoperative calcitonin levels is a key indicator of the prognosis: if less than 6 months, there is a 25% 5-year survival and 8% 10-year survival. Between 6 and 24 months, 97% and 32% respectively, and greater than 24 months, there were no deaths in the study.
More specifically the prognosis at each stage is as follows: Stage I - 100%, Stage II - 93%, Stage III - 71% and Stage IV - 21%.
Follow-up
Patients with medullary cancer need life-long follow-up, including regular monitoring of the calcitonin level in the blood. MTC often becomes chronic and only slowly progressive, so that patients without a cure tend to live with the disease for many years.
Following surgery long-term thyroid replacement is essential. Unlike follicular (FTC) and papillary (PTC) cancers there is no need to suppress the TSH below the normal level.
External beam radiation (EBRT) may be considered if there is evidence of gross or microscopic residual cancer in the neck, as it reduces local recurrence, but it has no effect on survival. Its potential tumour effect has to be weighed against the significant side-effects of the radiation. It is also useful in managing brain and bony metastases.
Because medullary cancer does not concentrate iodine, radioactive iodine is ineffective.
Ideally the surgery will remove the entire thyroid and all the lymph nodes in the neck which harbour metastatic spread. In this case, the post operative calcitonin will be undetectable, but this regrettably is a rare event and calcitonin levels often remain elevated, although less than before.
Calcitonin monitoring
The calcitonin and CEA levels correlate well with the tumour burden in the patient. Ideally after surgery both will return to undetectable levels, but if abnormal the level of calcitonin can direct the next steps.
A calcitonin doubling time of less than 6 months indicates a poorer prognosis. A patient with a rising calcitonin level, but still at a level less than 400, is going to have some residual disease, but is unlikely to have metastases detectable on imaging. In this case it is probably better to observe for a while, and repeat the calcitonin test later.
When the calcitonin level is more than 400 (evidence of either a recurrence or extension of persisting disease), a variety of imaging tests will need to be done to assess the site of disease (ultrasound of neck, CT of neck, chest and abdomen, MRI or FDG-PET scanning). Despite a rising calcitonin it is more often than not that these tests are negative, and in such circumstances a wait and see policy is also appropriate.
If there is good evidence of removable local disease in the neck it is best removed, although beneficial effect on prognosis is unproven. Even though parathyroid or adrenal tumours have already been removed it must be remembered that these tumours can be multiple and can reappear at a later date. Repeat blood and urine tests must be done on a regular basis.
The other thing to remember is that even though the medullary cancer recurrence may not be removable by surgery, many patients have very slow-growing, indolent disease and can often live with metastases for many years, with minimal symptoms.
Management of Metastatic Disease
If the disease recurs and cannot be removed surgically, this opens the possibility of systemic therapies, however the first thing to do is assess the tempo of the disease process (calcitonin doubling time, changes in disease burden over time, etc.).
Systemic therapy should not be administered to patients who have increasing serum calcitonin and CEA levels but no documented metastatic disease. Nor should systemic therapy be administered to patients with stable low-volume metastatic disease, as determined by imaging studies and serum calcitonin and CEA doubling times greater than 2 years.
It may be possible to consider the following:
- palliative local options e.g. radiotherapy to a bony metastasis, lung resection, etc.
- local hepatic measures e.g. embolisation
- antiresorptive drugs for bone health
- drugs to control diarrhoea, pain and anorexia
- tyrosine kinase inhibitor drugs (TKI's)
- these have significant side effects so should only be used when the patient has a high tumour burden and the consequences of not treating are worse than the drug toxicities.
- lenvatinib has response rates of 30%, others are vandetanib and cabozantinib, but all have toxicities of diarrhoea, rash, hypertension with headache, fatigue and nausea. - palliative cytotoxic chemotherapy e.g. 5FU, DTIC, etc.
- potential peptide receptor radionuclide therapies e.g. if FDG-PET positive