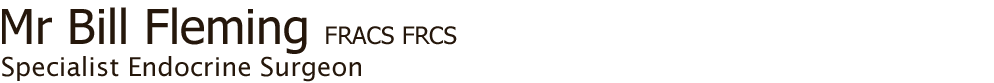Papillary Cancer (PTC) Management
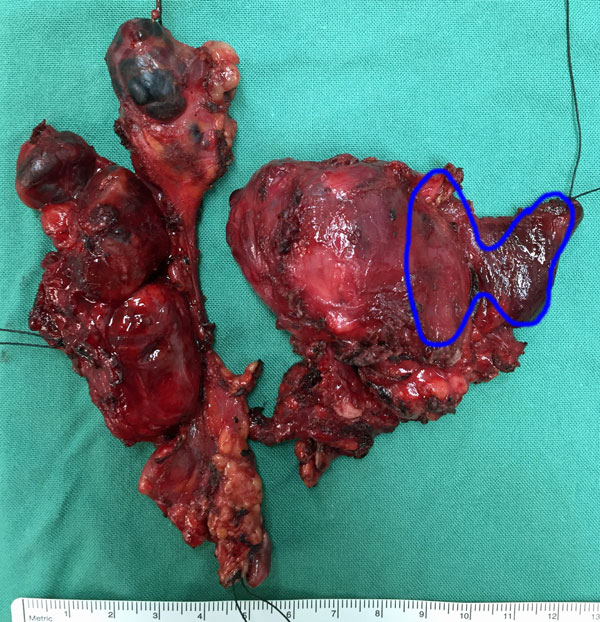 Fig. 1: Total thyroidectomy specimen containing PTC, plus involved nodes from right neck; the normal size of thyroid is superimposed over the specimen
Fig. 1: Total thyroidectomy specimen containing PTC, plus involved nodes from right neck; the normal size of thyroid is superimposed over the specimen
The management of papillary thyroid cancer (PTC) follows certain principles and has the following aims:
- remove all of the tumour and any nodes involved
- minimise morbidity (complications)
- stage the disease
- facilitate postoperative therapies e.g: radioactive iodine, thyroxine suppression
- to evaluate the response to therapy (dynamic risk stratification)
- permit long-term surveillance
- minimise the risk of recurrence or the development of metastases
Although the details have changed a little over the last few years, and radioiodine can often be omitted in some cases, PTC management is generally achieved by a four-pronged attack:
- Thyroid Surgery
- Radioactive iodine therapy
- Drug Treatment - Thyroxine therapy
- Regular follow-up
Tumour size at diagnosis has decreased from an average of 20 to 15mm over the last 20 years, but also treatment has become much more conservative, in both the extent of surgery, and the use of radioiodine therapy (RAI).
A ‘‘less is more’’ approach to larger, well-differentiated thyroid cancers (DTC) is increasingly accepted, particularly for NIFTP, encapsulated Follicular Variant of PTC (FV-PTC), and even classical PTC, up to 4 cm in diameter, though this size threshold remains controversial in Australia.
However, there remains an important role for total thyroidectomy for higher-risk thyroid cancers, where adjuvant therapy with radioactive iodine (RAI) plays an important role. Those tumours with higher risk have:
- invasive growth
- extrathyroidal extension
- vascular invasion
- bulky or extensive lymph node involvement
- distant metastatic spread, where adjuvant therapy with radioactive iodine (RAI) continues to play an important role.
There are many tumours that can now be managed by hemithyroidectomy (lobectomy) alone, and there is a much more selective use of radioiodine. In the postoperative followup there is less reliance on RAI scans, more use of ultrasound, and thyroglobulin can be used even without thyroid ablation with RAI.
When assessing the quality of thyroid cancer management, higher volume surgeons (who perform a lot of thyroid surgery each year, like me) have better outcomes in successfully removing cancers, and have a lower complication rate.
Recent research also suggests that the percentage thyroid remnant uptake (on postoperative radioactive iodine scanning after total thyroidectomy) and the lymph node ratio (number of involved nodes over total nodes removed) are directly related to the risk of thyroid cancer recurrence and mortality. The higher the uptake, or ratio, the higher the risk of recurrence.
I have created a booklet outlining the steps in diagnosis and treatment of thyroid cancer, which can be found on webpage Guide to Surgery.
Thyroid surgery
Patients with thyroid cancer will generally be recommended to have surgery for their cancer, but there are exceptions possible. Those patients who have very low risk cancers like papillary microcarcinoma (PMTC), or who are unfit for surgery, or who have medical conditions that mean a very short expected life-span, can be managed without surgery and only undergo active surveillance.
In practice however, almost all patients will be offered surgery, even those with PMTC, due to the small percentage of these patients who present with clinically significant regional (lymph node) or distant metastases.
The details of the surgery are covered in the webpages on Thyroid surgery, but the primary object of surgery is to remove all the cancer in the thyroid, plus any disease in the local lymph nodes or involved local structures (Fig. 1). Completeness of surgical resection is an important determinant of the outcome, while residual metastatic lymph nodes represent the most common site of disease persistence/recurrence.
The American Thyroid Association has recently published new guidelines in 2015 for the management of thyroid cancer and the associated lymph nodes, which in the case of papillary thyroid cancer, can be summarised below:
- for patients with thyroid cancer >4 cm, or with gross extrathyroidal extension (clinical T4), or clinically apparent metastatic disease to nodes (clinical N1) or distant sites (clinical M1), the initial surgical procedure should include a near-total or total thyroidectomy and gross removal of all primary tumour.
- for patients with thyroid cancer >1 cm and <4 cm without extrathyroidal extension, and without clinical evidence of any lymph node metastases (cN0), the initial surgical procedure can be either a total thyroidectomy or a lobectomy. Thyroid lobectomy alone may be sufficient initial treatment for low-risk papillary and follicular carcinomas; however, in practice the treatment team will generally choose total thyroidectomy to enable RAI therapy or to enhance followup based upon disease features and/or patient preferences.
- if surgery is chosen for patients with thyroid cancer <1 cm, without extrathyroidal extension and cN0, the initial surgical procedure should generally be a thyroid lobectomy. This is sufficient treatment for small, unifocal, intrathyroidal carcinomas, provided there are no higher risk factors, such as prior head and neck radiation, familial thyroid carcinoma, or clinically detectable cervical nodal metastases.
- all children with papillary thyroid cancer and a history of neck irradiation should be treated by total thyroidectomy.
- completion thyroidectomy should be offered to patients for whom a bilateral thyroidectomy would have been recommended had the diagnosis been available before the initial surgery.
- therapeutic central-compartment (level VI) neck dissection for patients with clinically involved central nodes should accompany total thyroidectomy to provide clearance of disease from the central neck.
- prophylactic central-compartment neck dissection (usually ipsilateral or occasionally bilateral) should be considered in patients with papillary thyroid carcinoma with clinically uninvolved central neck lymph nodes (cN0) who are considered high risk, such as male sex, age >45 years, advanced primary tumours with extracapsular or extrathyroidal disease (T3 or T4) or clinically involved lateral neck nodes (cN1b).
- there is no advantage of prophylactic central compartment neck dissection in low risk disease. Thyroidectomy without prophylactic central neck dissection is appropriate for small (T1 or T2), noninvasive, clinically node-negative PTC (cN0) and for most follicular cancers.
- therapeutic lateral neck compartmental lymph node dissection should be performed for patients with biopsy-proven metastatic lateral cervical lymphadenopathy.
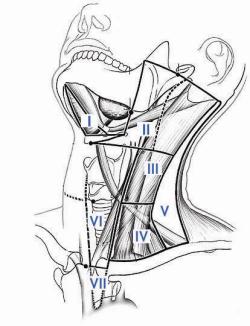 Fig. 2: Lymph node levels of the neckManagement of Lymph Nodes (see also Lymph Nodes in Thyroid Cancer)The most commonly involved central lymph nodes in thyroid carcinoma are the prelaryngeal (Delphian), pretracheal, and the right and left paratracheal nodal basins (Level VI). The AJCC TNM classification system classifies PTC with central compartment nodes as N1a, but if lateral nodes are involved the classification is raised to N1b. This correlates with the risk of recurrence in the two groups.
Fig. 2: Lymph node levels of the neckManagement of Lymph Nodes (see also Lymph Nodes in Thyroid Cancer)The most commonly involved central lymph nodes in thyroid carcinoma are the prelaryngeal (Delphian), pretracheal, and the right and left paratracheal nodal basins (Level VI). The AJCC TNM classification system classifies PTC with central compartment nodes as N1a, but if lateral nodes are involved the classification is raised to N1b. This correlates with the risk of recurrence in the two groups.
Generally lymph node involvement begins at the central compartment and then spreads to the lateral compartment. It is possible however, for papillary cancer to skip the central compartment nodes and appear first in the lateral compartment, in as much as 20-35% of patients. This is usually in level III or II, and is more commonly associated with upper pole tumours, single primary tumours and small (<1cm) tumours. However, skip metastases are unpredictable and their clinical significance is not clear.
If the recurrent laryngeal nerve is surrounded by lymph nodes it can usually be preserved, as PTC nodes tend to encase the nerve rather than invade it.
A central neck dissection includes comprehensive, compartment-oriented removal of the prelaryngeal and pretracheal nodes and at least one paratracheal lymph node basin (Fig. 2). A designation should be made as to whether a unilateral or bilateral dissection is performed and on which side (left or right) in unilateral cases.
Lymph node ‘‘plucking’’ or ‘‘berry picking’’ implies removal only of the clinically involved nodes rather than a complete nodal group within the compartment and is not recommended.
A therapeutic central compartment neck dissection implies that nodal metastasis is apparent clinically (preoperatively or intraoperatively) or by imaging (clinically N1a). A prophylactic, elective central compartment dissection implies nodal metastasis is not detected clinically or by imaging (clinically N0).
Initial Response to Therapy
The 2015 ATA Thyroid Cancer Guidelines recommend that after initial therapy patients response to the therapy should be evaluated to help guide the next steps in management, such as RAI or other therapies. While initial staging systems provide important insights into an individual patient’s risk of recurrence and disease-specific mortality, they provide static, single-point estimates of risk based only on data available at the time of initial therapy.
None of the currently available initial staging systems are capable of using new data obtained during the course of follow-up to modify the initial risk estimate. An individual patient's risk of recurrence can and does change over time. For example, an ATA low-risk patient with a rising serum Tg and neck nodes highly suspicious for recurrent disease at some point during follow-up would still be classified as ATA low risk, despite the presence of clinical data demonstrating a high risk of recurrence.
Conversely, an ATA high-risk patient that remains without evidence of disease over 30 years of appropriate follow-up would still be classified as ATA high risk despite having a risk of recurrence that is significantly lower than would have been predicted at the time of initial therapy.
Therefore, the initial staging systems should be used to guide initial management recommendations, and then a response-to-therapy assessment during follow-up can be used to modify these initial risk estimates in an ongoing, dynamic process.
As originally conceived by Mike Tuttle, these response-to-therapy clinical outcomes described the best response to initial therapy during the first 2 years of follow-up, but they are now being used to describe the clinical status at any point during follow-up (Fig. 3). They are:
- Excellent response: no clinical, biochemical, or structural evidence of disease
- Biochemical incomplete response: abnormal Tg or rising anti-Tg antibody levels in the absence of localisable disease.
- Structural incomplete response: persistent or newly identified loco-regional or distant metastases
- Indeterminate response: nonspecific biochemical or structural findings that cannot be confidently classified as either benign or malignant. This includes patients with stable or declining anti-Tg antibody levels without definitive structural evidence of disease.
 Fig. 3: A simplified overview of the clinical implications of the response-to-therapy reclassification system in patients treated with total thyroidectomy and RAI ablation, based on the research from Tuttle. Source: 2015 ATA Thyroid Cancer Guidelines
Fig. 3: A simplified overview of the clinical implications of the response-to-therapy reclassification system in patients treated with total thyroidectomy and RAI ablation, based on the research from Tuttle. Source: 2015 ATA Thyroid Cancer Guidelines
Multiple studies have now shown that many patients initially classified as intermediate or high risk of recurrence using initial staging systems can be reclassified later into a low risk of recurrence, based on having an excellent response to initial therapy.
Radioactive Iodine
Information for patients
It 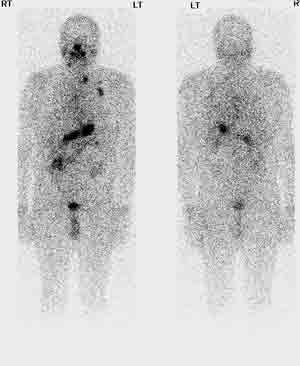 Fig. 4: Whole body scan prior to radioactive iodine treatmentis very difficult to remove absolutely all the thyroid tissue in the neck when performing a "total thyroidectomy", but the remnant tissue should be kept to an absolute minimum (see above) to lower the risk of recurrence.
Fig. 4: Whole body scan prior to radioactive iodine treatmentis very difficult to remove absolutely all the thyroid tissue in the neck when performing a "total thyroidectomy", but the remnant tissue should be kept to an absolute minimum (see above) to lower the risk of recurrence.
Radioactive iodine is used to destroy any remaining normal thyroid tissue in the neck or malignant thyroid tissue that was not able to be removed. It is not given to patients only having half the thyroid removed as it is ineffective. The advantage of radioactive iodine is that it acts as a ‘targeted treatment’ because thyroid tissue (and thyroid cancer tissue) selectively takes up iodine from the blood stream.
By attaching a radioactive particle to the iodine molecule, this allows a targeted dose of radiation to treat only the thyroid cells, including any malignant thyroid tissue provided the TSH level is elevated, with little damage to the surrounding structures.
Radioactive iodine is used in three separate situations:
- After total thyroidectomy surgery to destroy any residual thyroid cancer cells or residual normal thyroid tissue
- To treat thyroid cancer that has spread to the lymph nodes, lungs or bones (Fig. 4)
- To treat thyroid cancer that has come back after initial treatment by surgery or previous radioactive iodine or both
Prior to treatment the blood TSH must be grossly elevated so there is maximum take up of the radioactive iodine by the thyroid cells. This can be done in two ways: the traditional method is to stop thyroxine replacement (T4) until the TSH is elevated, although this takes 3-4 weeks, and can make the patient feel very weak and tired near the end of this time.
To avoid this problem it is now possible to instead stay on thyroxine and have an injection of artificial TSH (rhTSH or ‘Thyrogen’) a day or so before the radioactive iodine, which has the same effect of raising the TSH level, and still allows an effective uptake of radioactive iodine by thyroid tissue.
Radioactive iodine can be a rather tedious treatment as it entails being isolated in a special lead-shielded room for up to 3 days until the radioactivity has dissipated, although most treatments require a much shorter time in isolation (Fig. 5). It is given by swallowing a special capsule, but is usually a one-off treatment, is painless, and you can take whatever you like into the room with you, such as your phone, iPad, laptop, etc.
There are few immediate complications, although there is sometimes tenderness in the neck or in the parotids (mumps glands). It is rarely severe however, and usually responds well to a short course of steroids. Later problems may include blockage of the parotid ducts with a mildly swollen face at meal times, which is usually self-limiting. It does not make you feel sick or make your hair fall out, and most patients have no complications. Fig. 5: Radioiodine room at the Austin Hospital
Fig. 5: Radioiodine room at the Austin Hospital
In particular, there is no evidence that radioactive iodine treatment results in other cancers later, nor is there evidence that RAI reduces fertility in the male or female.
It is considered good practice for females not to become pregnant until at least six months after treatment, and males should follow the same rule, delaying pregnancy in their partners for at least six months.
Information for doctors
De-escalation of therapy has become a dominant theme in the use of RAI to treat DTC. Nevertheless, higher-risk, follicular cell–derived thyroid cancers remain candidates for adjuvant therapy with RAI, despite a worldwide move to more selective use of this treatment modality.
Critically, there should be a clear distinction between the use of RAI to treat known or likely residual cancer, and the use of RAI to ablate a (normal) thyroid remnant. The latter can be achieved when necessary using low-dose (30 mCi) doses, with uptake stimulated by recombinant human thyrotropin (TSH: Thyrogen), but this is unnecessary if criteria defining successful ablation have already been achieved by surgery alone.
In higher-risk cases, however, RAI remains an important tool for adjuvant therapy, with compelling data for efficacy in selected cases, albeit exclusively retrospective in nature. There remains significant controversy around the precise level of risk of recurrence or mortality at which RAI use is justified.
There is considerable excitement about the potential for re-differentiation therapy in RAI-refractory DTC, using inhibitors of the MEK pathway, which are in active clinical trials. Short-term use of such drugs to facilitate effective RAI treatment abrogates many of the concerns about the cumulative toxicity of systemic therapy for thyroid cancer and holds the tantalizing promise of effective treatment for unresectable locoregional disease and RAI-refractory metastatic disease.
When assessing the quality of thyroid cancer management, higher volume surgeons (who perform a lot of thyroid surgery each year) have better outcomes in successfully removing cancers, and have a lower complication rate. Recent research suggests that the percentage thyroid remnant uptake (on postoperative radioactive iodine scanning) and the lymph node ratio (number of involved nodes over total nodes removed) are directly related to the risk of thyroid cancer recurrence and mortality. The higher the uptake, or ratio, the higher the risk of recurrence.
Radioiodine administration in its first dose is given according to the level of risk of recurrence (see response to therapy (above) and the webpage Staging & Risk), and has three main functions:
1. RAI Remnant Ablation
To facilitate initial staging, and allow the detection of recurrent disease during followup, by tests such as:
- thyroglobulin
- whole body scanning
2. RAI Adjuvant Therapy
By theoretically destroying suspected, but unproven residual disease, especially in patients at increased risk of disease recurrence, leading to:
- increased survival rate
- lower recurrence rate
3. RAI Therapy
Given to higher-risk patients with persistent or recurrent disease to improve disease-specific and disease-free survival
According to the 2015 American Thyroid Association Guidelines, consideration should be given to specific features of the individual patient such as comorbidities, the logistics of disease follow-up, and patient preference when making a decision about RAI.
In general radioiodine use should follow these guidelines:
- ATA Low risk - tumour <1cm (uni or multifocal): not recommended
- ATA Low risk - tumour 1-4cm: not routinely recommended, unless aggressive histology or vascular invasion
- ATA Low-Intermediate risk - tumour >4cm, microscopic extrathyroid extension, or cervical nodal metastases: consider, especially if adverse features (older age, large size or number of nodes, lateral nodes)
- ATA High risk - gross extrathyroid extension, distant metastases: recommended
For all higher-risk patients, such as those with known distant metastases, gross extrathyroidal extension regardless of tumour size, or primary tumour size greater than 4cm, even in the absence of other high-risk features, RAI ablation is considered standard management because: large residual thyroid beds with high uptake may obscure metastases, serum thyroglobulin (Tg) is a better marker of recurrence after RAI ablation, and retrospective studies show a reduced incidence of tumour recurrence and death.
Alternatively, RAI is not recommended in any patient with tumour size less than 1cm, without higher risk features. In this very low-risk group, particularly in microcarcinomas, surgery will effect a cure and radioactive iodine is unnecessary. The cancer-specific mortality rate is 1% at 30 years. This group should not be considered for re-operative surgery if a lobectomy was initially performed.
Radioiodine ablation is recommended for selected patients with thyroid cancers 1-4cm in size, confined to the thyroid, documented lymph node metastases or other higher risk features, when the combination of age, tumour size, lymph node status and individual histology puts the patient in an intermediate or high risk group.
Recent research has demonstrated that low dose RAI (~30mCi) is just as effective as higher dose RAI (~75-150 mCi) in ablation of all thyroid remnants. Posttreatment scans are obtained on the third day, and if the patient’s activity falls to permitted levels, he or she is discharged with advice by the medical physicist regarding contact with other people.
After 4 months the thyroglobulin is measured and a repeat total-body scan may be performed, although much less likely now, unless there was significant uptake at post-therapy scan.
If successful ablation is achieved after the first dose of RAI, no further treatment is necessary and the patient is followed with regular thyroglobulin estimation, and a routine neck ultrasound at 12 months post treatment. Lifetime follow-up is mandatory.
If initial ablation is not successful, remnant ablation will need to be performed at 6- to 12-monthly intervals until ablation is complete. The definition of complete ablation is arbitrary. A negative scan with an undetectable thyroglobulin clearly represents total ablation. Patients with negative scans or little uptake, but detectable thyroglobulins, do not benefit from a further dose of RAI. These patients should be watched and given further ablation therapy if there is a significant rise in the thyroglobulin.
When the scan is clear and the thyroglobulin is undetectable, further whole body scanning is unnecessary and thyroglobulin alone is used as a tumour marker. Patients with a negative scan and significantly high thyroglobulins should have ablation therapy, as often their post-therapy scan will highlight residual disease.
The management of patients with persistent disease following RAI treatment is difficult. It is important to remember that RAI is often ineffective in ablating nodal disease. Persistent disease arises due to incomplete tumour resection or aggressive disease. Wherever possible, the surgical option of excising residual tissue should be followed, as this aids subsequent RAI therapy. When recurrence is suspected, re-staging is performed using ultrasound, CT, magnetic resonance imaging (MRI) or positron emission-tomography (PET) scanning.
Thyroxine therapy
The final part of the treatment is to suppress the TSH by giving thyroxine, which has the effect of preventing regrowth of any thyroid cells (see webpage Thyroxine Treatment). Benefits of low TSH have been shown, especially in high risk groups, with decreased thyroid cancer recurrence and thyroid cancer-related death. Thyroxine suppression is only given to patients who have undergone a total thyroidectomy, and not to those who have had only lobectomy (partial thyroidectomy).
In the low risk patient the aim is to get the TSH down to the low normal range (0.1-0.5 mU/l), but in high risk patients more aggressive suppression is required, down to well below the normal range (<0.1 mU/l). The initial dose of thyroxine is decided according to body weight, and thereafter adjusted according to the results of the blood tests of thyroid function. The first TSH check is usually done at about 6-8 weeks after treatment and the dose of thyroxine adjusted up or down as necessary.
Low risk groups can have their TSH suppression gradually wound back after some years, to hold the TSH in the low normal range, to prevent effects on cardiovascular and bone health. In those with persistent disease, TSH suppression should be maintained below 0.1 mU/l indefinitely in the absence of specific contraindications. Those in high risk groups, but who have had successful ablation and are clinically and biochemically free of disease can end TSH suppression after 5-10 years.
Follow-up
While most patients are cured by their treatment, thyroid cancer can recur or spread to other parts of the body, even many years after surgery. That is why regular follow up checks are needed for life, especially in the first 5-10 years after surgery when the risk of the cancer returning is highest.
There are a number of tests that are used to monitor progress:
- Thyroglobulin blood test
- Thyroid function test (to monitor thyroxine replacement levels)
- Ultrasound
- occasionally, Whole body scan
1. Followup after lobectomy
Followup after thyroid lobectomy for low risk PTC is more problematic than after the more extensive treatment of PTC with total thyroidectomy and radioiodine ablation. There is an excellent disease-specific survival and highly sensitive disease detection techniques are unnecessary.
A practical approach is as follows:
- TSH goal: aim for 0.5-2.5 mIU/ml, with or without thyroxine
- followup visits
- postop for wound check, pathology results
- 6-8 weeks for check of thyroid function and thyroglobulin baseline
- 6-12 months for clinical exam
- yearly for 2-3 years for exam
- TSH, T4, thyroglobulin and TGAB at each visit
- imaging
- neck US at 6-12 months, then 2 years and 5 years
- then rarely unless indicated
- late completion thyroidectomy
- physical findings
- US findings
- need for RAI
- sustained rise in thyroglobulin levels
2. Followup after Total Thyroidectomy and RAI
Thyroglobulin blood test
A thyroglobulin (Tg) test measures the level of thyroglobulin in the blood. It is generally performed once or twice a year during follow up, unless it is abnormal, when more frequent estimation may be done.
Thyroglobulin is a protein in the blood that stores thyroid hormone. Because thyroid cells (and thyroid cancer cells) are the only cells in the body that make thyroglobulin it is a very accurate measure of whether thyroid cancer cells are present somewhere in the body. If the Tg is elevated, then investigation by ultrasound or whole body scan is used to check for the source of the excess thyroglobulin.
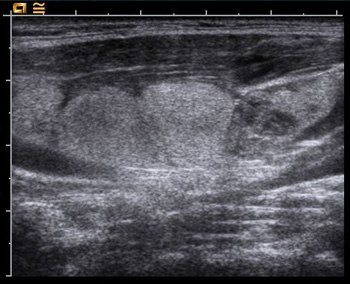 Fig. 6: US showing enlarged lymph nodes containing thyroid cancerTg is usually measured while the patient is still taking thyroid tablets (so that the TSH is still suppressed), but occasionally it can be necessary to perform a ‘stimulated Tg’ by doing the test while the TSH is elevated. This is achieved by either stopping the thyroid tablets for a few weeks, or by using recombinant human TSH (rhTSH) or ‘Thyrogen’.
Fig. 6: US showing enlarged lymph nodes containing thyroid cancerTg is usually measured while the patient is still taking thyroid tablets (so that the TSH is still suppressed), but occasionally it can be necessary to perform a ‘stimulated Tg’ by doing the test while the TSH is elevated. This is achieved by either stopping the thyroid tablets for a few weeks, or by using recombinant human TSH (rhTSH) or ‘Thyrogen’.
Thyroid function tests are regularly performed to make sure that the amount of thyroxine tablets is sufficient to replace the function of the thyroid and to suppress the TSH below the normal range, to minimise the risk of recurrence of the cancer.
Ultrasound
An ultrasound of the neck is usually done once a year in the first few years as a routine, looking for recurrence of the cancer and enlargement of lymph nodes (Fig. 6), which might indicate spread of the cancer.
It also allows for a FNA biopsy to be done of anything suspicious, including measuring the thyroglobulin level in the nodes (thyroglobulin washout).
In low risk patients the combination of stimulated thyroglobulin measurement and high resolution neck ultrasound has a 97-98% sensitivity to detect disease and 95-96% negative predictive value. Those low risk patients with undetectable thyroglobulin and negative ultrasound do not require further routine whole body scanning during followup.
High or intermediate risk patients may derive some value from a further whole body diagnostic scan at 6-12 months after ablation.
Other Tests
Occasionally other tests besides the ultrasound will be required, especially if there is evidence of recurrence.
A whole body scan (WBS) requires the patient to swallow a pill or liquid that contains a small amount of radioactive iodine and then lie down under a large camera that takes a scan of the whole body. If any thyroid or thyroid cancer cells are present they will show up as ‘hot spots’ on the film.
PET scanning is another form of whole body scanning using sophisticated nuclear medicine techniques, which is very effective in locating thyroid cancer cells. It is usually only used when there is evidence of disease on other tests.