Adrenocortical Carcinoma
This is a rare malignant tumour of the adrenal cortex, which is the most malignant of the endocrine tumours and carries a grave prognosis. Although potentially curable by surgery at an early stage, only 30% of patients have a tumour confined to the adrenal gland at the time of diagnosis.
The incidence of adrenocortical cancer (ACC) is only 0.5-2 cases per million per year, usually twice as commonly in women, with a mean age at presentation of 40-50 years. The cause of the tumour is not well understood, and involves a variety of genetic mutations.
Presentation
1. Hormone excess
Approximately 60% of patients with ACC present with symptoms related to hormone hypersecretion, such as rapidly progressive Cushing's syndrome or virilisation. Cushing's syndrome is the most common hormonal presentation (50-80%), but it is important to remember that less than 10% of Cushing's syndrome is caused by ACC.
Virilisation secondary to excess androgens is the second most common hormonal presentation, with rapid-onset male pattern baldness, hirsutism, virilisation, and menstrual irregularities in women. Oestrogen production occurs in 1% to 3% of male ACC patients, causing gynaecomastia and testicular atrophy.
2. Mass syndrome
About 30% don't have a functioning tumour and will usually present with symptoms related to the growth of the tumour. Patients complain of pain in the abdomen, flank or back, often with associated nausea, vomiting or abdominal fullness. Weight loss and loss of appetite are common.
Metastases can occur within the abdomen to liver, but also to lungs and bones.
3. Incidentalomas
The remaining 10 - 20% have ACC that is found during the investigation and treatment of an adrenal incidentaloma (usually larger than 4cm in size).
Investigation
Hormone Excess
Initial investigation will be by blood and urine testing looking for excess adrenal hormones being produced by the tumour. The majority of ACCs produce too much hormone (cortisol, testosterone, oestrogen, aldosterone, etc.), but of course this is also a feature of most benign tumours and hyperplasia of the adrenals.
Malignant tumours however tend to have extremely high levels of circulating hormones. Thus it is not always possible to distinguish between benign and malignant (cancerous) tumours on the basis of hormones alone.
Investigations should include: serum cortisol & ACTH +/- low dose dexamethasone suppression test, sex hormones & DHEAS, plasma metanephrines and aldosterone renin ratio.
Imaging
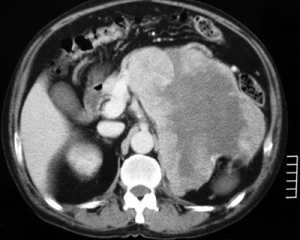 Fig.1: Left adrenocortical cancerThe best imaging technique is CT scan of the tumour, but MRI and PET scanning also have useful benefits in determining the extent of tumour and invasion of surrounding structures.
Fig.1: Left adrenocortical cancerThe best imaging technique is CT scan of the tumour, but MRI and PET scanning also have useful benefits in determining the extent of tumour and invasion of surrounding structures.
The size of the adrenal tumour is one of the best indicators of malignancy:
- <4cm 2% risk
- 4-6cm 6% risk
- >6cm 25% risk of ACC
ACC tends to be large, with irregular margins, and typically are heterogeneous in texture due to haemorrhage, necrosis and calcification (Fig. 1). They may have evidence of local invasion or tumour extension, and may also show evidence of metastases.
Measurement of Hounsfield units in unenhanced CT can be very helpful in distinguishing benign from malignant tumours, with lower HU levels indicating benign pathology. Using a cutoff level of 10HU is highly sensitive for detecting benign tumours; as HU levels rise above 10HU the risk of malignancy also increases.
Investigation of blood supply by angiography may be required before surgery is undertaken.
All imaging should be done as close as possible to the operation date as some aggressive tumours can grow quickly, changing the potential operability.
Adrenal biopsy
This is controversial due to the risk of tumour spillage, needle track metastases and the difficulty of distinguishing benign from malignant tumours. It will generally only be used in the case of adrenal metastases, or if surgery is not feasible in the case of a suspected ACC prior to medical treatment. It also must only be performed after exclusion of a phaeochromocytoma.
Staging
At the time of presentation, ACCs are generally large tumours, measuring on average 10 to 13 cm. Only a minority of tumours are <6 cm (9%–14%), with only 3% presenting as lesions <4 cm.
The ENSAT (European Network for the Study of Adrenal Tumours) staging system has been widely adopted recently, as it better reflects patient outcomes per stage than the UICC system. The ENSAT staging system defines 4 stages. Stage 1 (≤5 cm) and stage 2 (>5 cm) tumours are confined to the adrenal gland. Stage 3 tumours extend into surrounding tissue (eg, para-adrenal adipose tissue or adjacent organs) or involve locoregional lymph nodes. Stage 4 is reserved for patients with distant metastasis.
The most common metastatic sites are lung (40%–80%), liver (40%–90%), and bone (5%–20%).
| Staging System | ||
|---|---|---|
| UICC/WHO | ENSAT | |
| Stage 1 | T1, N0, M0 | T1, N0, M0 |
| Stage 2 | T2, N0, M0 | T2, N0, M0 |
| Stage 3 | T1–2, N1, M0 | T1–2, N1, M0 |
| T3, N0, M0 | T3–4, N0, M0 | |
| Stage 4 | T1–4, N0–1, M1 | T1–4, N0–1, M1 |
| T3–4, N1, M0 | ||
| T4, N0, M0 | ||
Abbreviations: UICC, International Union Against Cancer; WHO, World Health Organization.
Tumours are classified as follows: T1, ≤5cm tumour; T2, >5cm tumour; T3, tumour infiltration into surrounding tissue; T4, tumour invasion into adjacent organs; N0, no positive lymph nodes; N1, positive lymph node(s); M0, no distant metastases; M1, presence of distant metastasis.
Treatment
Currently, the only curative approach to ACC is complete tumour resection at surgery. Adjuvant therapies aim to decrease the chance of recurrence, but all therapy of unresectable or metastatic ACC must be considered palliative.
Surgery
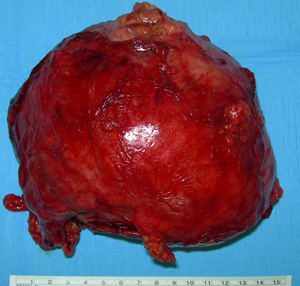 Fig.2: Specimen of a large adrenocortical carcinomaPreoperative imaging should be obtained to evaluate the extent of tumour, possible invasion of surrounding anatomical structures, and the feasibility of the tumour to be completely resected.
Fig.2: Specimen of a large adrenocortical carcinomaPreoperative imaging should be obtained to evaluate the extent of tumour, possible invasion of surrounding anatomical structures, and the feasibility of the tumour to be completely resected.
Imaging studies also help to guide the surgeon as to the expected extent of resection required. Careful attention should be paid to adjacent organs, the adrenal and renal veins, the inferior vena cava, and the aorta, including the takeoff of the coeliac and superior mesenteric arteries.
Despite preoperative diagnostic tests, approximately 25% of stage 3 cases are initially suspected to be stage 2 ACC but ultimately found to have microscopic extension through the adrenal capsule.
Imaging should be obtained as close as possible to the anticipated date of surgery, because many aggressive ACCs grow quickly and involvement of adjacent structures may change, thereby altering the operative plan.
Preoperatively, management and optimization of those patients with hormone excess should be undertaken, especially those with Cushing's syndrome, due to the numerous deleterious effects of elevated cortisol (poor wound healing, infection, and metabolic derangements). Aggressive control of cortisol excess should be attempted in the short period between identification of the tumour and surgery, but surgery should not be unnecessarily delayed solely to tightly control hypercortisolism.
Adrenalectomy by open operation is the method of choice, including any involved surrounding structures such as the kidney (Fig. 2). However complete resection is rarely possible, as only 30% of patients present with the tumour confined to the adrenal gland, and many have microscopic spread at the time of diagnosis.
Pathological diagnosis can be difficult in many cases. The Weiss score was devised in 1984 to try and help distinguish between benign and malignant adrenal tumours, which even after removal can be difficult to separate.
Weiss described a combination of nine criteria that he found most useful in distinguishing malignant from benign tumours: nuclear grade III or IV; mitotic rate greater than 5/50 high-power fields; atypical mitoses; clear cells comprising 25% or less of the tumour; a diffuse architecture; microscopic necrosis; and invasion of venous, sinusoidal, and capsular structures.
None of the 24 tumours in his study with two or less of these criteria metastasised or recurred, while all but one of the 19 tumours with four or more of these criteria either recurred or metastasised.
Surgery for local recurrence or metastatic disease may also be possible and may improve survival.
Medical therapy
In general, those with widespread distant metastatic disease in multiple organs or those with multiple metastatic deposits in one organ system, unable to be completely resected, should not undergo adrenalectomy. The primary tumour can instead be treated with external beam radiation for palliation along with other adjuncts to improve local symptoms and better control hormone excess, if present.
Radiotherapy may be useful in local recurrence. External beam radiotherapy can be useful for local control.
Chemotherapy protocols will usually include the drug mitotane, which is derived from the insecticide DDT, the only adrenal-specific agent available for treatment of ACC. It is an inhibitor of steroid synthesis, and toxic to adrenal cortical cells. It does induce adrenal insufficiency and requires steroid replacement, but often causes toxic side effects.
The efficacy of mitotane therapy in the setting of not completely resectable, metastasised, or recurrent ACC is well established. Overall, 30% of patients show stable disease or partial remission after treatment with mitotane in this setting.
Chemotherapy with other combinations of drugs like cisplatin and etoposide may be given, but only a minority of patients will respond.
Hormonal therapy with steroid synthesis inhibitors such as ketoconazole, metyrapone and aminoglutethimide may be used in a palliative manner to reduce the symptoms of hormonal syndromes.
Survival
Despite the generally unfavorable prognosis of ACC, there is a marked individual variation in disease progression, recurrence, and overall survival. Even in patients with stage 4 disease, survival ranges from a few months to several years.
Unfortunately the prognosis for patients with ACC is poor, with only 20% of patients surviving 5 years and a median survival of less than 12 months.